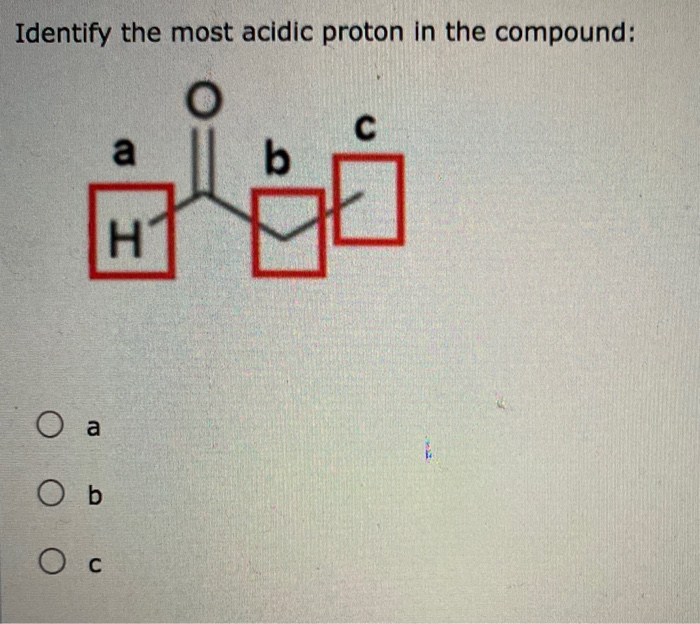
Thus, Proton C, situated between the carbonyls, is our most acidic proton. Common Functional Groups with the Corresponding pKa Values To build on our discussion, let’s delve into the factors determining the acidity of protons: resonance, atomic size, electronegativity of atoms carrying a negative charge, inductive effects, and hybridization.
The following compounds are listed in increasing order of acidity… | Channels for Pearson+
question No one rated this answer yet — why not be the first? 😎 FarzaLC Explanation: In this case, among the indicated protons, the most acidic one is option a) II. The answer is option–>a) II Explanation: a) II: This proton is attached to a carbon atom that is part of an aromatic ring.
Source Image: chegg.com
Download Image
To give you the short answer: An acidic solution has a high concentration of hydrogen ions (H + ), greater than that of pure water. A basic solution has a low H + concentration, less than that of pure water. To see where this definition comes from, let’s look at the acid-base properties of water itself. Autoionization of water

Source Image: youtube.com
Download Image
Most acidic proton: first semester organic chemistry The lone pair on an amine nitrogen, by contrast, is not part of a delocalized p system, and is very ready to form a bond with any acidic proton that might be nearby. Often it requires some careful thought to predict the most acidic proton on a molecule. Ascorbic acid, also known as Vitamin C, has a pK a of 4.1.
Source Image: chegg.com
Download Image
Which Of The Indicated Protons Is Most Acidic
The lone pair on an amine nitrogen, by contrast, is not part of a delocalized p system, and is very ready to form a bond with any acidic proton that might be nearby. Often it requires some careful thought to predict the most acidic proton on a molecule. Ascorbic acid, also known as Vitamin C, has a pK a of 4.1. Step 1 1 of 2 To know which of the indicated protons is the most acidic we need to know which of the conjugate base will be the most stable. Proton A is called an aryl proton – deprotonation leads to the formation of a really unstable aryl anion which quickly reacts to regain protonation state.
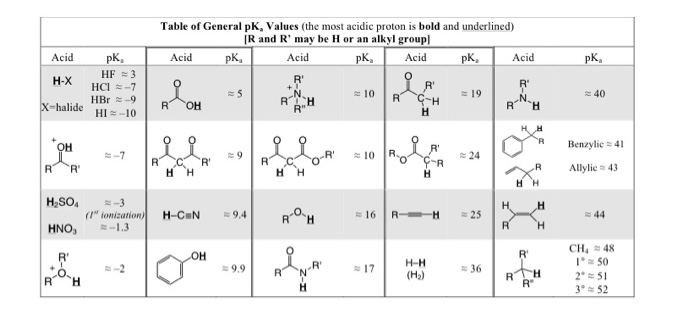
Matching Use the pka chart (provided on D2L under | Chegg.com
Jul 29, 2022How to: There are three general methods to estimate the acidity of a proton: 1) Use the table of common acids that we learned in class and is in your book (Table 3.1, inside front cover of the book), and try to either find the same acidic proton or similar one in the table. 2) Identify which functional group the proton is a part of like: alkane Determine, based on the pKa values, if each of the following compounds can be protonated by water. | Organic reactions, Organic chemistry, Chemistry

Source Image: pinterest.com
Download Image
Aluminum-substituted phosphotungstic acid/sulfonated poly ether ether ketone nanocomposite membrane with reduced leaching and improved proton conductivity – Soma Banerjee, Kamal K Kar, 2016 Jul 29, 2022How to: There are three general methods to estimate the acidity of a proton: 1) Use the table of common acids that we learned in class and is in your book (Table 3.1, inside front cover of the book), and try to either find the same acidic proton or similar one in the table. 2) Identify which functional group the proton is a part of like: alkane

Source Image: journals.sagepub.com
Download Image
The following compounds are listed in increasing order of acidity… | Channels for Pearson+ Thus, Proton C, situated between the carbonyls, is our most acidic proton. Common Functional Groups with the Corresponding pKa Values To build on our discussion, let’s delve into the factors determining the acidity of protons: resonance, atomic size, electronegativity of atoms carrying a negative charge, inductive effects, and hybridization.

Source Image: pearson.com
Download Image
Most acidic proton: first semester organic chemistry To give you the short answer: An acidic solution has a high concentration of hydrogen ions (H + ), greater than that of pure water. A basic solution has a low H + concentration, less than that of pure water. To see where this definition comes from, let’s look at the acid-base properties of water itself. Autoionization of water
Source Image: physicsforums.com
Download Image
Dark or light roasts? What’s better for your stomach? – Henry’s House Of Coffee The lone pair on an amine nitrogen, by contrast, is not part of a delocalized p system, and is very ready to form a bond with any acidic proton that might be nearby. Often it requires some careful thought to predict the most acidic proton on a molecule. Ascorbic acid, also known as Vitamin C, has a pK a of 4.1.

Source Image: henryshouseofcoffee.com
Download Image
Carbon Dioxide Reacts With Water – LabXchange The lone pair on an amine nitrogen, by contrast, is not part of a delocalized p system, and is very ready to form a bond with any acidic proton that might be nearby. Often it requires some careful thought to predict the most acidic proton on a molecule. Ascorbic acid, also known as Vitamin C, has a pK a of 4.1.

Source Image: labxchange.org
Download Image
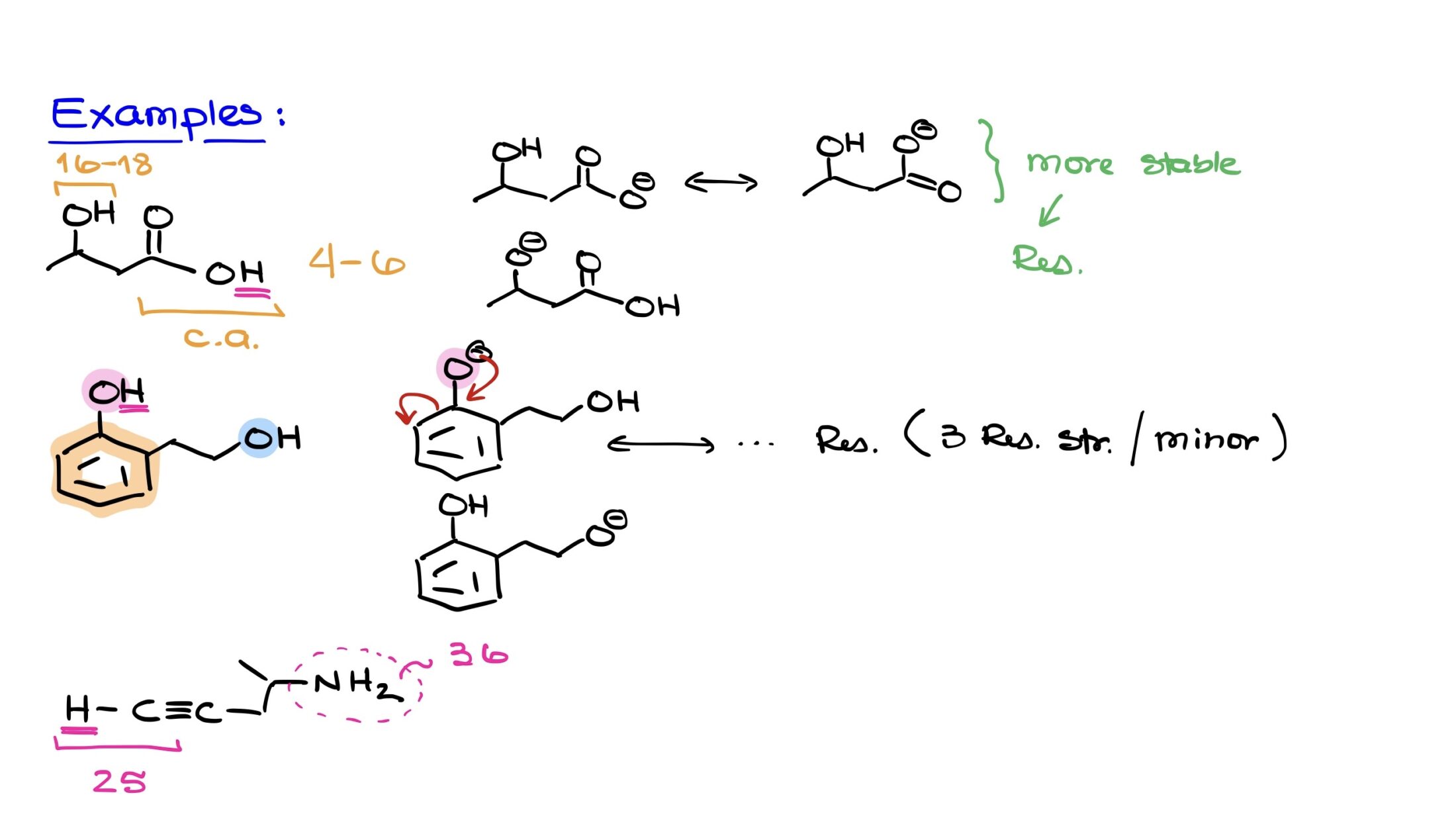
How to Find the Most Acidic Proton in a Molecule — Organic Chemistry Tutor Step 1 1 of 2 To know which of the indicated protons is the most acidic we need to know which of the conjugate base will be the most stable. Proton A is called an aryl proton – deprotonation leads to the formation of a really unstable aryl anion which quickly reacts to regain protonation state.
Source Image: organicchemistrytutor.com
Download Image
Aluminum-substituted phosphotungstic acid/sulfonated poly ether ether ketone nanocomposite membrane with reduced leaching and improved proton conductivity – Soma Banerjee, Kamal K Kar, 2016
How to Find the Most Acidic Proton in a Molecule — Organic Chemistry Tutor question No one rated this answer yet — why not be the first? 😎 FarzaLC Explanation: In this case, among the indicated protons, the most acidic one is option a) II. The answer is option–>a) II Explanation: a) II: This proton is attached to a carbon atom that is part of an aromatic ring.
Most acidic proton: first semester organic chemistry Carbon Dioxide Reacts With Water – LabXchange The lone pair on an amine nitrogen, by contrast, is not part of a delocalized p system, and is very ready to form a bond with any acidic proton that might be nearby. Often it requires some careful thought to predict the most acidic proton on a molecule. Ascorbic acid, also known as Vitamin C, has a pK a of 4.1.