The nuclear reaction can be written as: 12 25 Mg + 2 4 He 1 1 H + Z A X. where A is the mass number and Z is the atomic number of the new nuclide, X. Because the sum of the mass numbers of the reactants must equal the sum of the mass numbers of the products: 25 + 4 = A + 1, or A = 28. Similarly, the charges must balance, so:
What Is an Elemental Analyzer & How Does It Work?
Apr 22, 2022So for this question we have been given a number of nuclear reactions and we need to find the particle that is emitted in these nuclear reactions. So first of all we should know how to represent an element. And for the … Complete these nuclear reactions with the particle that is emitted. {H _ {A+ 0 GgCu – 8zn+LJ 28QPo L7 ZPb+ 0J Answer Bank

Source Image: study.com
Download Image
Complete these nuclear reactions with the particle that is emitted. GH – {#+OJ Answer Bank #Cu + It + 6Zn + #4Po 205pb. Instant Answer. Step 1/3 1. First, we need to identify the given nuclear reactions. … Complete these nuclear reactions with the particle that is emitted. {H _ {A+ 0 GgCu – 8zn+LJ 28QPo L7 ZPb+ 0J. Answer Bank. 01:55.

Source Image: radiopaedia.org
Download Image
SOLVED: Complete these nuclear reactions with the particle that is emitted: H -> H + 1n SSCu -> Szn + 28QPo 283Pb -> Science Chemistry Chemistry questions and answers Complete these nuclear reactions with the particle that is emitted. 12H 11H+2961Cu 3061Zn+84210Po 82206 Pb+ This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: pubs.acs.org
Download Image
Complete These Nuclear Reactions With The Particle That Is Emitted.
Science Chemistry Chemistry questions and answers Complete these nuclear reactions with the particle that is emitted. 12H 11H+2961Cu 3061Zn+84210Po 82206 Pb+ This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Jun 4, 2023The following are the completed nuclear reactions with the particle that is emitted.In the above nuclear reaction, an alpha particle (a) is emitted.A nuclear reaction is a process that alters the nucleus of an atom, resulting in a change in the atom’s composition.
Chemical Looping Combustion in a Packed Fluidized Bed Reactor─Fundamental Modeling and Batch Experiments with Random Metal Packings | Energy & Fuels
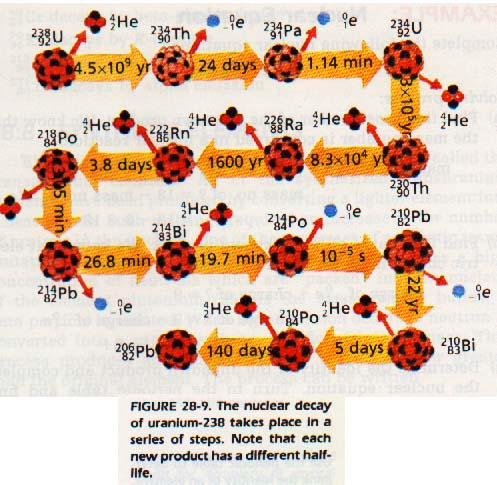
Jan 5, 2023Has a proton and a neutron. has no neutrons and only one proton.Consequently, a neutron will be the additional particle on the product side. What kind of particle does this nuclear process produce? The proton stays in the nucleus while the electron is released as a particle, increasing the atomic number without changing the mass number.. With an example, what is nuclear reaction? Ch 28
Source Image: boomeria.org
Download Image
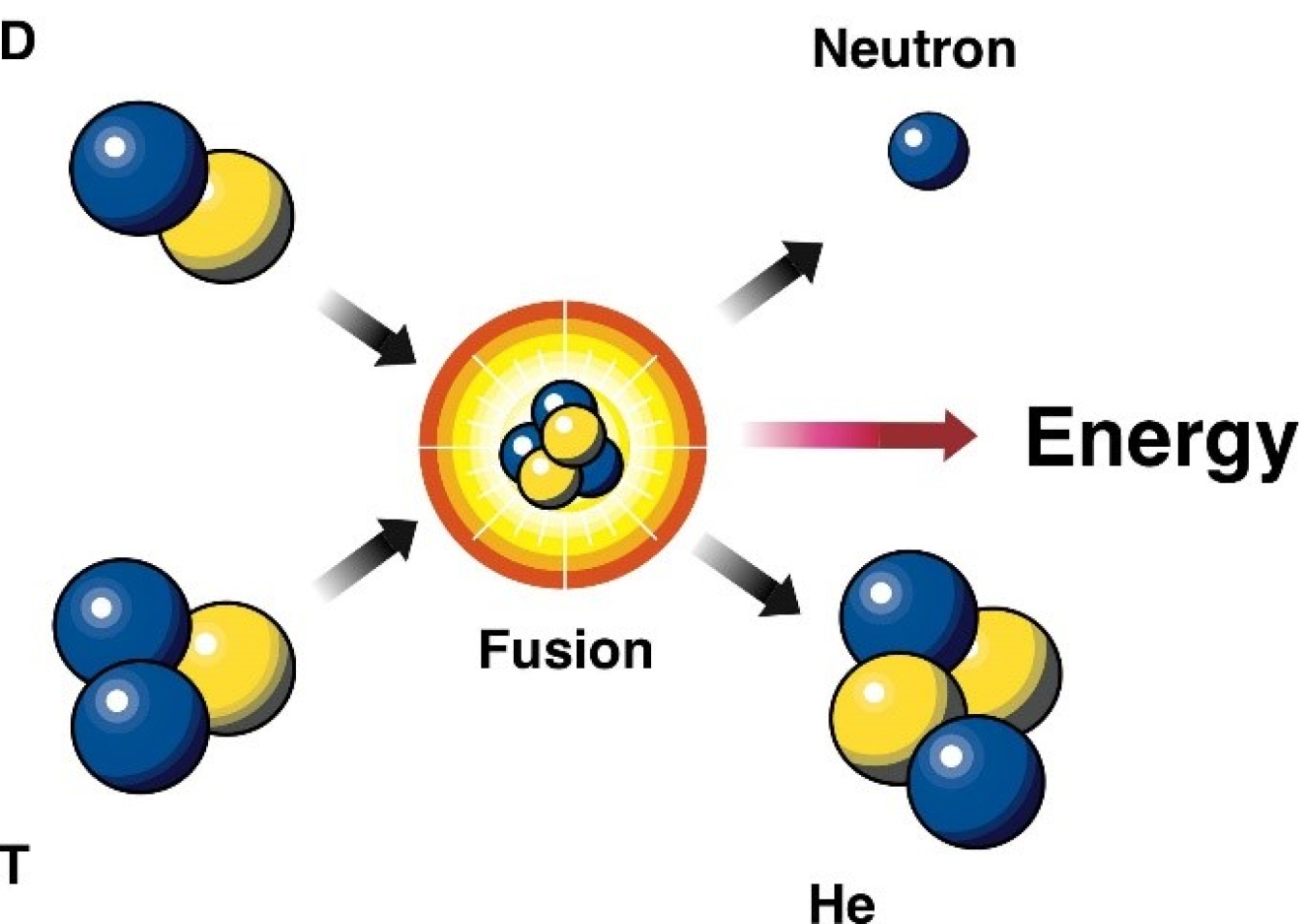
DOE Explains…Nuclear Fusion Reactions | Department of Energy Jan 5, 2023Has a proton and a neutron. has no neutrons and only one proton.Consequently, a neutron will be the additional particle on the product side. What kind of particle does this nuclear process produce? The proton stays in the nucleus while the electron is released as a particle, increasing the atomic number without changing the mass number.. With an example, what is nuclear reaction?
Source Image: energy.gov
Download Image
What Is an Elemental Analyzer & How Does It Work? The nuclear reaction can be written as: 12 25 Mg + 2 4 He 1 1 H + Z A X. where A is the mass number and Z is the atomic number of the new nuclide, X. Because the sum of the mass numbers of the reactants must equal the sum of the mass numbers of the products: 25 + 4 = A + 1, or A = 28. Similarly, the charges must balance, so:

Source Image: excedr.com
Download Image
SOLVED: Complete these nuclear reactions with the particle that is emitted: H -> H + 1n SSCu -> Szn + 28QPo 283Pb -> Complete these nuclear reactions with the particle that is emitted. GH – {#+OJ Answer Bank #Cu + It + 6Zn + #4Po 205pb. Instant Answer. Step 1/3 1. First, we need to identify the given nuclear reactions. … Complete these nuclear reactions with the particle that is emitted. {H _ {A+ 0 GgCu – 8zn+LJ 28QPo L7 ZPb+ 0J. Answer Bank. 01:55.
 Download Image
Download ImageSOLVED: Complete these nuclear reactions with the particle that is emitted. Zu H + Answer Bank ^63Cu + ^1H → ^64Zn + ^1n + 2^4He + ^208Pb Oct 10, 2022The answer is nuclear radioactivity, that is, high-energy particles produced in radioactive decays heat Earth from the inside (Figure 7.5.6 ). Figure 7.5.6: Earth is heated by nuclear reactions (alpha, beta, and gamma decays). Without these reactions, Earth’s core and mantle would be much cooler than it is now.

Source Image: numerade.com
Download Image
Positrons in Nuclear Medicine | Open Medscience Science Chemistry Chemistry questions and answers Complete these nuclear reactions with the particle that is emitted. 12H 11H+2961Cu 3061Zn+84210Po 82206 Pb+ This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
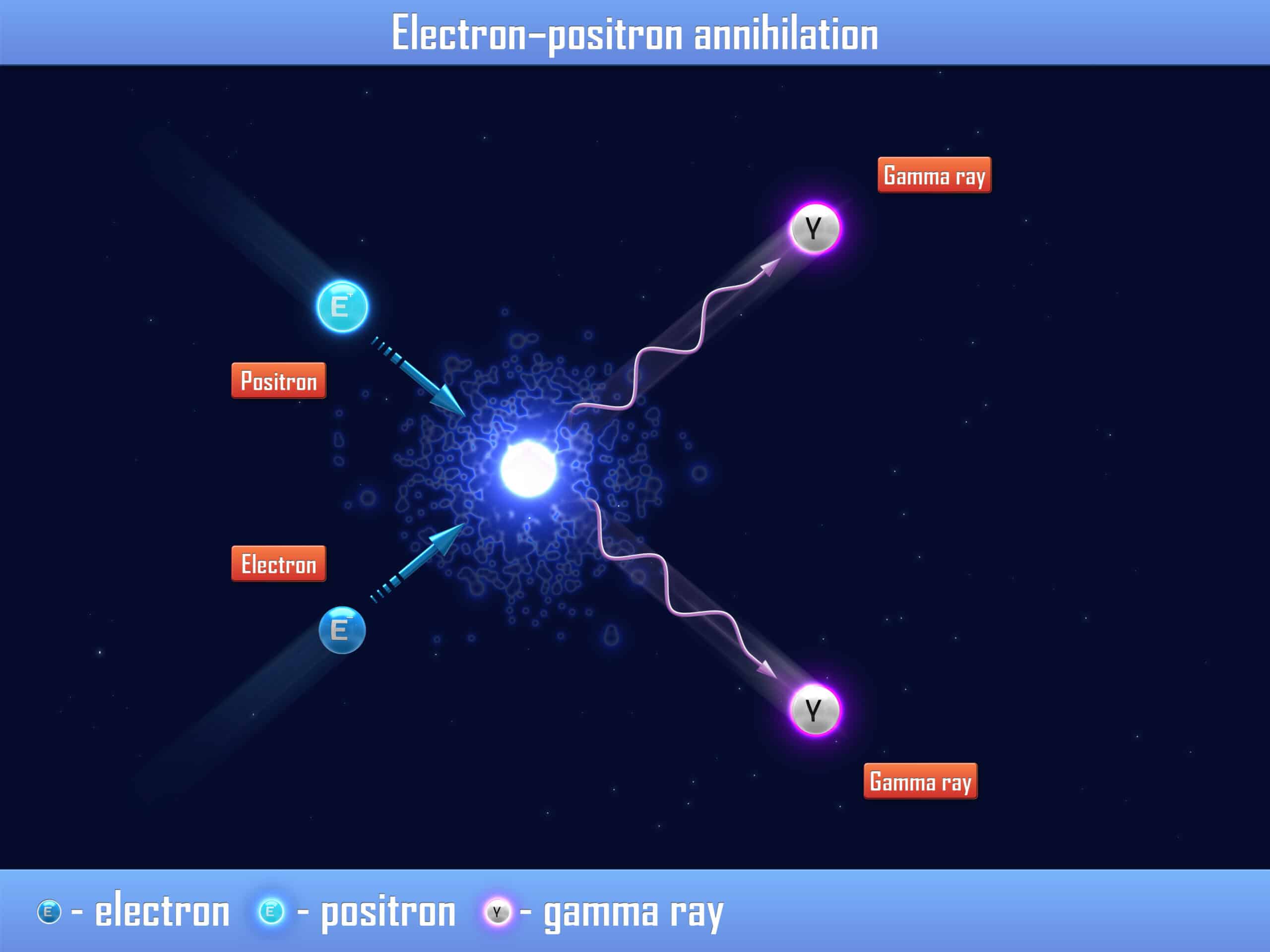
Source Image: openmedscience.com
Download Image
How to Write Nuclear Equations for Gamma Decay | Chemistry | Study.com Jun 4, 2023The following are the completed nuclear reactions with the particle that is emitted.In the above nuclear reaction, an alpha particle (a) is emitted.A nuclear reaction is a process that alters the nucleus of an atom, resulting in a change in the atom’s composition.

Source Image: study.com
Download Image
DOE Explains…Nuclear Fusion Reactions | Department of Energy
How to Write Nuclear Equations for Gamma Decay | Chemistry | Study.com Apr 22, 2022So for this question we have been given a number of nuclear reactions and we need to find the particle that is emitted in these nuclear reactions. So first of all we should know how to represent an element. And for the … Complete these nuclear reactions with the particle that is emitted. {H _ {A+ 0 GgCu – 8zn+LJ 28QPo L7 ZPb+ 0J Answer Bank
SOLVED: Complete these nuclear reactions with the particle that is emitted: H -> H + 1n SSCu -> Szn + 28QPo 283Pb -> Positrons in Nuclear Medicine | Open Medscience Oct 10, 2022The answer is nuclear radioactivity, that is, high-energy particles produced in radioactive decays heat Earth from the inside (Figure 7.5.6 ). Figure 7.5.6: Earth is heated by nuclear reactions (alpha, beta, and gamma decays). Without these reactions, Earth’s core and mantle would be much cooler than it is now.