May 26, 2023And Why? May 26, 2023 by Jay Rana The common Ionic Charges of Copper (Cu) are 1+ and 2+. But the question is how can you find the ionic charge on Copper (Cu)? Well, copper is a transition element and all the transition and post-transition elements have a variable ionic charge.
IJERPH | Free Full-Text | Removal of Copper Ions from Wastewater: A Review
Apr 17, 2023Solution. CuO. Cu2O. Write the name leaving room for a Roman numeral, since copper forms ions with variable charges. copper ( ) oxide. copper ( ) oxide. Based on position on the periodic table, the oxide ion has a charge of 2-. Note that CuO has only one copper ion, while Cu 2 O has two copper ions. Both have only one oxide ion.

Source Image: nagwa.com
Download Image
In this case, the copper atom carries a positive charge. Cu – e – → Cu +. Here, the electron configuration of copper ion (Cu +) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This copper ion (Cu +) has twenty-nine protons, thirty-five neutrons, and twenty-eight electrons. Also, copper has one more ion.

Source Image: pubs.acs.org
Download Image
Safe, fast-charging lithium battery handles 5 times the current Jun 30, 2023Occurrence and extraction of Copper. Copper occurs both in combined state and free state and also in many ores. The important ores of copper are copper pyrites ( CuFeS2 C u F e S 2 ), cuprite and copper glance. The copper ores are mostly found in the north of India. The extraction of copper also involves many steps.

Source Image: pubs.acs.org
Download Image
What Is The Charge Of A Copper Ion
Jun 30, 2023Occurrence and extraction of Copper. Copper occurs both in combined state and free state and also in many ores. The important ores of copper are copper pyrites ( CuFeS2 C u F e S 2 ), cuprite and copper glance. The copper ores are mostly found in the north of India. The extraction of copper also involves many steps. Sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Image credit: Wikipedia Commons, public domain. Atoms are electrically neutral because the number of protons, which carry a 1+ charge, in the nucleus of an atom is equal to the number of electrons, which carry a 1- charge, in the atom.
Spectroscopic Methods in Bioinorganic Chemistry: Blue to Green to Red Copper Sites | Inorganic Chemistry
Jul 21, 2022Saying an ion has variable charges means that the same element can form ions with different charges. For example, it is possible for copper to form both an ion with a +1 charge and to form an ion with a +2 charge. The large majority of transition metals form ions with variable charges. There is also a small block of main group metals that form g-factor dependence on the effective nuclear charge of copper ion Z eff… | Download Scientific Diagram

Source Image: researchgate.net
Download Image
IJMS | Free Full-Text | Subcellular Localization of Copper—Cellular Bioimaging with Focus on Neurological Disorders Jul 21, 2022Saying an ion has variable charges means that the same element can form ions with different charges. For example, it is possible for copper to form both an ion with a +1 charge and to form an ion with a +2 charge. The large majority of transition metals form ions with variable charges. There is also a small block of main group metals that form

Source Image: mdpi.com
Download Image
IJERPH | Free Full-Text | Removal of Copper Ions from Wastewater: A Review May 26, 2023And Why? May 26, 2023 by Jay Rana The common Ionic Charges of Copper (Cu) are 1+ and 2+. But the question is how can you find the ionic charge on Copper (Cu)? Well, copper is a transition element and all the transition and post-transition elements have a variable ionic charge.
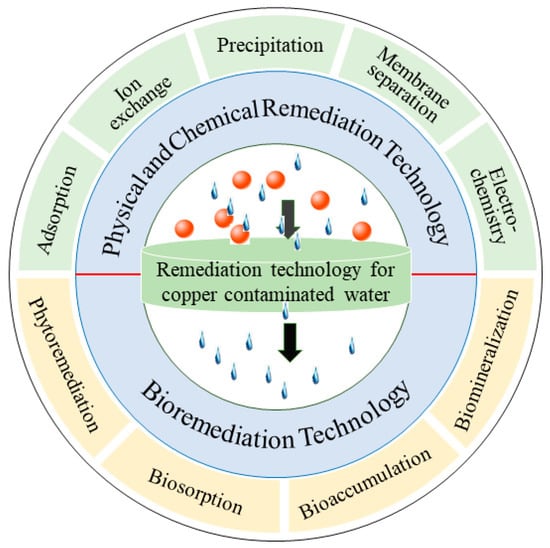
Source Image: mdpi.com
Download Image
Safe, fast-charging lithium battery handles 5 times the current In this case, the copper atom carries a positive charge. Cu – e – → Cu +. Here, the electron configuration of copper ion (Cu +) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This copper ion (Cu +) has twenty-nine protons, thirty-five neutrons, and twenty-eight electrons. Also, copper has one more ion.

Source Image: newatlas.com
Download Image
ToughSF: Nuclear EFP and HEAT Answer In its ionic state, copper (Cu) can have either a +1 or a +2 charge. Explanation: Copper is a d-block element, or a transition metal. This means that it can: exhibit variable valency due to valence electrons in two shells instead of one form ions that can achieve stability with incompletely filled d orbitals

Source Image: toughsf.blogspot.com
Download Image
savvy-chemist: Transition Metals: Complex Ions Jun 30, 2023Occurrence and extraction of Copper. Copper occurs both in combined state and free state and also in many ores. The important ores of copper are copper pyrites ( CuFeS2 C u F e S 2 ), cuprite and copper glance. The copper ores are mostly found in the north of India. The extraction of copper also involves many steps.
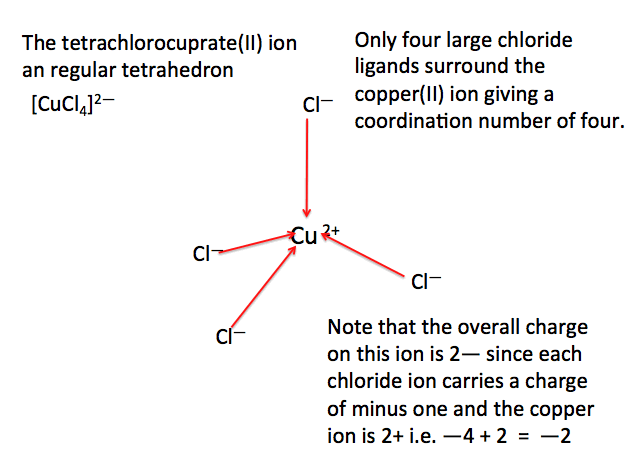
Source Image: derekcarrsavvy-chemist.blogspot.com
Download Image
Science With Screens: Experiment 61: Single-Transistor Ion Chamber for Detecting Radiation Sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Image credit: Wikipedia Commons, public domain. Atoms are electrically neutral because the number of protons, which carry a 1+ charge, in the nucleus of an atom is equal to the number of electrons, which carry a 1- charge, in the atom.
Source Image: sciencewithscreens.blogspot.com
Download Image
IJMS | Free Full-Text | Subcellular Localization of Copper—Cellular Bioimaging with Focus on Neurological Disorders
Science With Screens: Experiment 61: Single-Transistor Ion Chamber for Detecting Radiation Apr 17, 2023Solution. CuO. Cu2O. Write the name leaving room for a Roman numeral, since copper forms ions with variable charges. copper ( ) oxide. copper ( ) oxide. Based on position on the periodic table, the oxide ion has a charge of 2-. Note that CuO has only one copper ion, while Cu 2 O has two copper ions. Both have only one oxide ion.
Safe, fast-charging lithium battery handles 5 times the current savvy-chemist: Transition Metals: Complex Ions Answer In its ionic state, copper (Cu) can have either a +1 or a +2 charge. Explanation: Copper is a d-block element, or a transition metal. This means that it can: exhibit variable valency due to valence electrons in two shells instead of one form ions that can achieve stability with incompletely filled d orbitals